Fine Heart Lab
Location and Contact Information
- Lab 212-305-2964
- Barry Fine bmf2002@cumc.columbia.edu
Principal Investigator
The Fine Heart Lab studies molecular cardiology, focusing on utilizing stem cells, engineered human heart tissue and transgenic mouse models to study cardiac disease for therapeutic discovery. As a main site of the NIH funded tissue engineering resource center, the lab's mission is to advance tissue engineering to improve disease modeling across all organs. Dr. Fine's active research areas include myocardial infarction, inherited cardiomyopathies, and druggable signaling pathways in heart cells to improve heart function.

Current Research Projects
Current research projects in our laboratory include:
Cardiomyocyte Resiliency
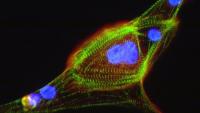
Much of heart disease is due to the unfortunate fact that cardiomyocytes (heart cells) do not regenerate after injury. During an event like a myocardial infarction (heart attack), the loss of significant numbers of cardiomyocytes leads to irreversible injury and heart failure. Our lab is focused on discovering druggable signaling pathways within cardiomyocytes that can decrease cell death during such events. In particular, we are focusing on new pathways that can control cell survival during periods of ischemia (low oxygen), which is the main driver of cell death during a heart attack. We have developed a novel bioreactor in which we use stem cell derived human heart cells to model a myocardial infarction and have identified the kinase STK25 as a master regulator of cardiomyocyte cell death. Several phosphorylation targets of STK25 were identified in human cardiomyocytes, including a regulatory subunit of PKA that leads to inhibition of adrenergic signaling. Currently, we are exploring novel small molecule inhibitors of STK25 in both human and mouse models of disease.
Stem Cell Models of Cardiomyopathies
One of the biggest impediments to scientific progress in heart disease has been a lack of a cell culture system with human cardiomyocytes. Now, circulating stem cells can be redirected towards generating human heart cells, providing a new system from which we can study cardiac biology in the laboratory. Coupled with advances in tissue engineering, the ability to generate mature cardiac muscle in the lab has led to a significant leap in our search for new cardiovascular therapies. In our lab, we utilize stem cells from patients with genetic diseases affecting the heart muscle (cardiomyopathies). We are interested in both exploring the genetic underpinnings of heart failure mechanistically as well as identifying new therapies that would positively impact their clinical course. We have demonstrated this pipeline by investigating restrictive cardiomyopathy, a rare disease with no therapies in which the heart becomes increasingly rigid to the point that the only recourse is heart transplantation. Our current research is focused on exploring how mutations in the sarcomeric protein Filamin C lead to different heart failure phenotypes and exploring new ways of gene therapy for patients with Filamin C mutations.
Collaborations
Dr. Fine serves as Co-Investigator for the Tissue Engineering Resource Center (TERC). The center serves as a place-to-go for established, junior, and aspiring investigators and trainees for bioreactors, software, materials, and protocols for tissue engineering research.
For additional information, please visit:
Social Media
For the latest developments, announcements, and updates, follow the Fine Heart Lab at Twitter/X: @fineheartlab
Lab Members
Select Publications
1. Teles, D., & Fine, B. M. (2024). Using induced pluripotent stem cells for drug discovery in arrhythmias. Expert Opinion on Drug Discovery, 19(7), 827–840. https://doi.org/10.1080/17460441.2024.2360420
2. Giangreco, N. P., Lebreton, G., Restaino, S., Farr, M., Zorn, E., Colombo, P. C., Patel, J., Soni, R. K., Leprince, P., Kobashigawa, J., Tatonetti, N. P., & Fine, B. M. (2022). Alterations in the kallikrein-kinin system predict death after heart transplant. Scientific Reports, 12(1), 14167. https://doi.org/10.1038/s41598-022-18573-2
3. Wang, B. Z., Nash, T. R., Zhang, X., Rao, J., Abriola, L., Kim, Y., Zakharov, S., Kim, M., Luo, L. J., Morsink, M., Liu, B., Lock, R. I., Fleischer, S., Tamargo, M. A., Bohnen, M., Welch, C. L., Chung, W. K., Marx, S. O., Surovtseva, Y. V., … Fine, B. M. (2023). Engineered cardiac tissue model of restrictive cardiomyopathy for drug discovery. Cell Reports Medicine, 4(3), 100976. https://doi.org/10.1016/j.xcrm.2023.100976
4. Zhang, X., Wang, B. Z., Kim, M., Nash, T. R., Liu, B., Rao, J., Lock, R., Tamargo, M., Soni, R. K., Belov, J., Li, E., Vunjak-Novakovic, G., & Fine, B. (2022). STK25 inhibits PKA signaling by phosphorylating PRKAR1A. Cell Reports, 40(7), 111203. https://doi.org/10.1016/j.celrep.2022.111203
View a complete list of our publications https://pubmed.ncbi.nlm.nih.gov/?term=Barry+Fine&sort=date









